Two teams of scientists have come up with creative new ways to help people with vision problems regenerate their own eyes.
In one experiment, researchers developed a new surgical technique that resets powerful stem cells in the eye to replace eye tissue damaged by cataracts and other eye diseases.
In another, a team of Japanese researchers found a way to turn ordinary skin cells into various types of cells found in the eye.
Both offer ways to eventually replace clumsy cataract or cornea surgery, outside experts said.
“These two studies illustrate the remarkable regenerative and therapeutic potential of stem cells,” ophthalmologist Dr. Julie Daniels of University College London wrote in a commentary in Nature, which published both studies.
Cataracts are the No. 1 cause of blindness worldwide: By age 80, more than half of Americans have a cataract or have had cataract surgery, according to the National Eye Institute. Up to 20 million people a year get the surgery globally.
“These two studies illustrate the remarkable regenerative and therapeutic potential of stem cells."
They’re caused when the proteins in the lens clump up, blurring and eventually often blocking vision. They are easily treated by removing the damaged lens and replacing it with a plastic lens, but it doesn’t always work and people can develop problems years later.
Some babies are born with cataracts, as well.
Researchers have been trying to develop ways to make stem cells, the body’s master cells, replace the damaged lens naturally.
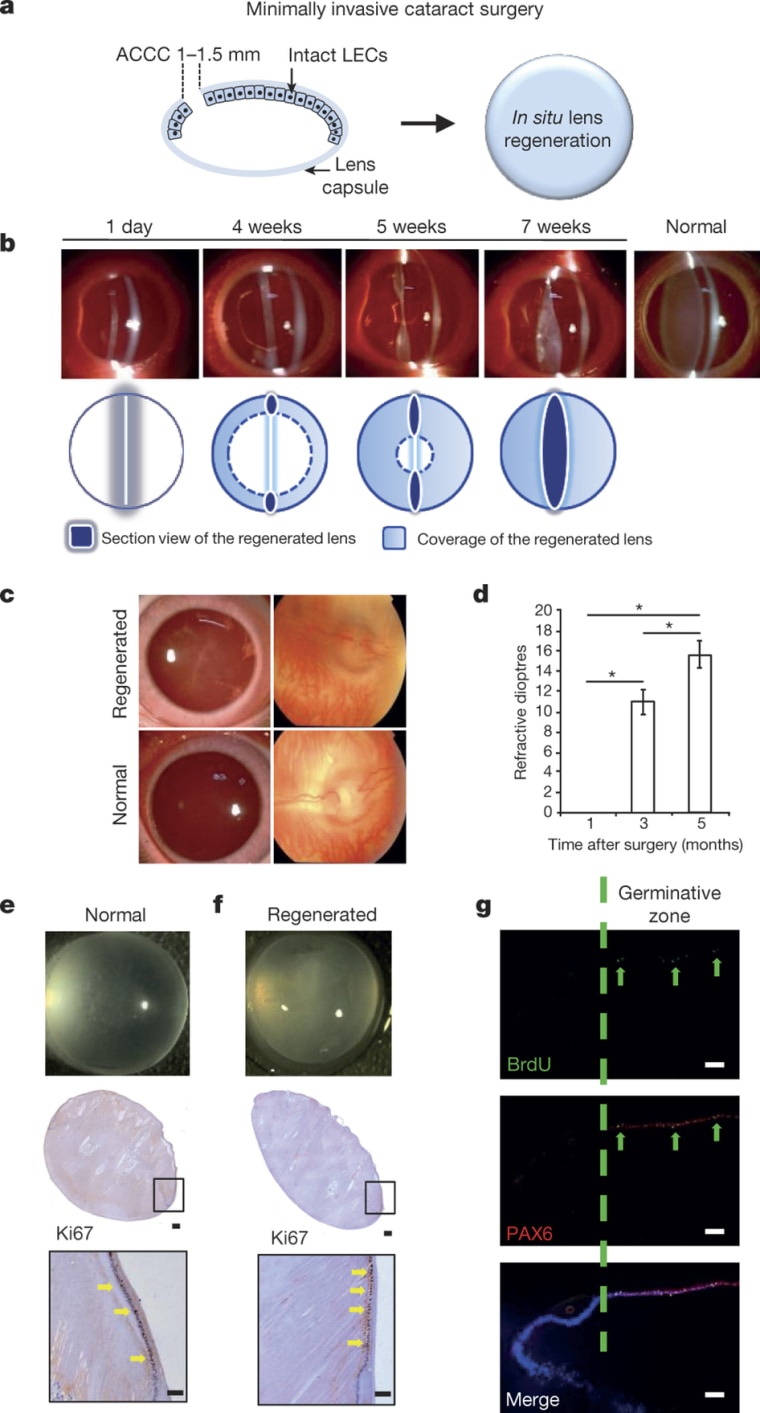
In the first study, Dr. Kang Zhang of Sun Yat-sen University of China and the University of California, San Diego, and colleagues tweaked a technique commonly used to remove the damaged lens so that more stem cells of a type called lens epithelial stem/progenitor cells would be left behind.
They noticed that stem cells sometimes proliferate when a damaged lens is removed, but not enough to regenerate the lens.
“The current surgical procedure for cataract treatment inadvertently destroys the integrity of the lens capsule and the very lens epithelial stem/progenitor cells that hold the regenerative key to lens restoration. It is also associated with numerous side-effects and a significant risk of complications, particularly in infants,” they wrote in their report, published in Nature.
They tried their method first in rabbits, then monkeys, and finally in 12 babies born with cataracts in China.
“We conducted a clinical trial in pediatric cataract patients up to two years of age to investigate whether lenses could be regenerated in humans using minimally invasive surgery,” they wrote.
There were no complications and all 12 children developed clear lenses. Their vision will be tested as they grow to see if it is normal.
“In this study, we show that a new minimally invasive cataract surgery method preserves the integrity of the lens capsule and associated lens epithelial stem/progenitor cells, facilitating functional lens regeneration in animals and humans,” the team wrote.
Several outside researchers praised the experiment.
Dr. Dusko Ilic, a stem cell scientist at King's College London, called it “one of the finest achievements in the field of regenerative medicine until now.”
“The basic science research led to the hypothesis that preserving and stimulating autologous stem cells in the eye might promote regeneration of a surgically removed lens. And indeed, their hypothesis was true. They proved it first by testing a new surgical approach in rabbits and primates before successfully treating 12 infants. It is science at its best,” Ilic said.
Such an approach may not work as well in adults, who have fewer stem cells in the eye, the researchers cautioned.
"It is science at its best."
But there may be a way to grow new eye parts.
Kohji Nishida of Osaka University Graduate School of Medicine in Japan and colleagues grew eye cells from induced pluripotent stem cells (iPS cells), which are made from ordinary cells such as skin cells. They are genetically or chemically tricked into becoming stem cells, and then can be re-directed to form the desired cell type.
They grew human iPS cells in a nourishing bath meant to trick them into acting as if they were developing eye cells. They did, forming four distinct zones consisting of different eye cell types.
Transplanted into rabbits, they grew into corneas. Now they want to try the method in humans.
“Corneal transplantation has been the gold standard for restoring transparency since the first successful transplant in 1905,” Daniels wrote in her comment.
“However, despite the fact that corneal transplants are less likely to induce an immune response than transplants to other sites in the body, grafts can be rejected by the host body within five years,” she added.
“As an alternative, the conversion of adult cells into induced pluripotent stem cells (iPSCs), which can develop into any cell type, could supply enough cells for therapy.”